What is the EMDN and why does it matter for your medical device?
Written by: Manuel Mateos CEO and Regulatory Affairs Director, CMC Medical Devices & Drugs S.L.
The EMDN code for medical devices is the official classification system adopted in the EU. It plays a key role in regulatory processes. As a result, it will be essential for registering products in EUDAMED once the database is fully operational.
What is the EMDN?
The European Commission developed the EMDN as a hierarchical system that assigns standardized codes to medical devices. It is based on the Italian CND (Classificazione Nazionale Dispositivi medici), a system that has been in use for many years. That’s why it’s considered a reliable and proven framework for identifying devices.
In contrast to other international nomenclatures, the EMDN is free to access and use. This makes it more accessible for manufacturers of all sizes and helps reduce costs during regulatory procedures.
Why you need the EMDN code for medical devices
You will need to assign an EMDN code for medical devices to your product when registering it in EUDAMED, once the module is fully functional.
This code:
- ensures consistency and traceability across the EU
- supports regulatory oversight and market surveillance
- allows Notified Bodies and Competent Authorities to properly identify your product
Choosing the right EMDN code for medical devices means going beyond the general category. You should always select the most specific term available for your product, based on its intended use and technology.
How to choose the right code
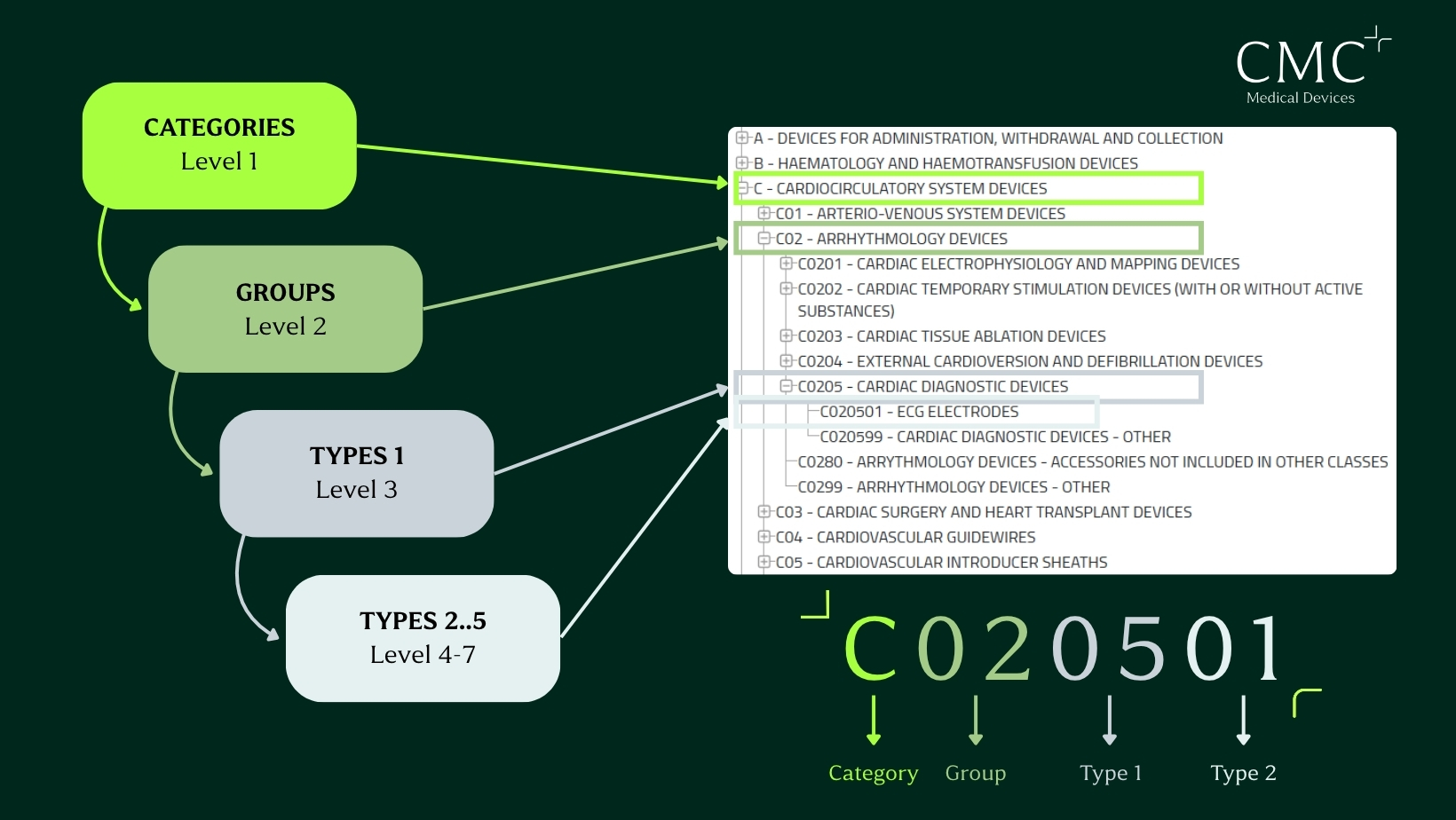
Start by browsing the official EMDN browser. The structure is divided into levels:
- Categories
- Groups
- Generic types
To avoid misclassification, the goal is to go as deep as possible into the tree structure. This helps you match your product accurately and avoid problems during audits or registration.
Example: ECG electrodes
- Category: C – Cardiocirculatory system devices
- Group: C0205 – Cardiac diagnostic devices
- Type: C020501 – ECG electrodes
Need help with the EMDN?
If your product doesn’t seem to fit any of the existing codes, or if you believe a new code should be created, the European Commission offers an EMDN helpdesk.
You can use this service mainly to submit proposals to expand the nomenclature. However, it is not designed to help with choosing your code.
Key takeaways
- The EMDN code for medical devices will be essential for EUDAMED registration
- It’s free, official, and based on a validated Italian model
- You should always choose the most specific code available
- An official helpdesk exists for submitting suggestions
Ready to ensure compliance with EUDAMED?
Make sure your EMDN code for medical devices is accurate. If you need support, we’re here to help you through every step of the process.