CE marking for Class I medical devices: Can I self-certify my product?
But is that really the case? In this article, we clarify the distinctions within Class I devices and help you understand whether your product qualifies for the simplified route, or if it requires external assessment.
What is a class I device and what does self-certification involve?
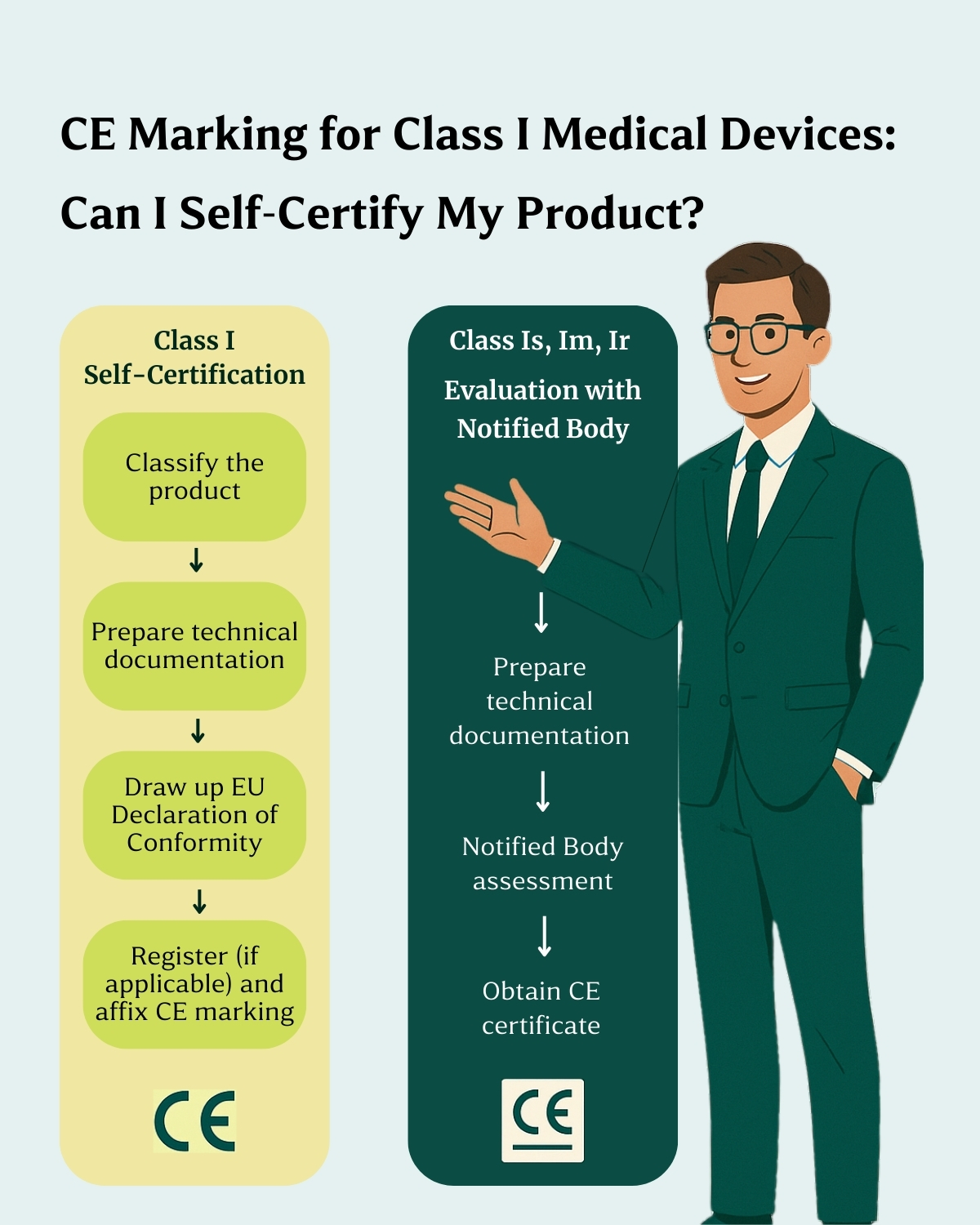
Under the Medical Device Regulation (MDR) 2017/745, Class I devices are considered low-risk and, in some cases, can be self-certified by the manufacturer.
This means the manufacturer is fully responsible for ensuring that the device meets all applicable safety and performance requirements, drafting the EU Declaration of Conformity, and affixing the CE mark.
Can all class I devices be self-certified?
No. Within Class I, there are specific subcategories that require Notified Body involvement, even though the devices are technically low-risk. These include:
- Sterile devices (Class Is)
- Devices with a measuring function (Class Im)
- Reusable surgical instruments (Class Ir)
In these cases, self-certification is not allowed. The manufacturer must submit the technical documentation for review by a Notified Body, who will evaluate compliance and issue the CE certificate accordingly.
How can I know if my device qualifies for self-certification?
To assess your product correctly, we recommend:
- Reviewing the product’s features in line with the MDR classification rules
- Confirming whether the device is sterile, measuring, or reusable
- Consulting regulatory experts to determine whether a Notified Body is required
What documents are needed for self-certifying a “Pure” class I device?
If your device is non-sterile, non-measuring, and not reusable, you may follow the self-certification route. In this case, you’ll need to:
- Draft the EU Declaration of Conformity (Annex IV of the MDR)
- Prepare the Technical Documentation (Annexes II and III)
- Implement a post-market surveillance (PMS) plan
- Register your device in EUDAMED (where applicable)
- Appoint an Authorized Representative in Europe if you are a non-EU manufacturer
Conclusion
While Class I devices benefit from a simplified CE marking process, not all can be self-certified. Understanding the differences within this category is crucial to avoid mistakes, penalties, or delays in bringing your device to the EU market.
Not sure whether your device qualifies for self-certification?
Contact us, we’ll analyze your product and guide you step by step through MDR compliance.